FDA registered medical instruments
Owner Operator Number: 10091625
Rehabsurge is now fully registered medical device establishment facility. EISTM tools are now fully registered Class 1 Medical devices. Please check FDA Registration and Device Listing Database with Registration Number: 3032912829


Exclusive Availability on Amazon
For those interested in purchasing the manual only, it is exclusively available on Amazon for $99. You can choose between a print edition or a Kindle version to suit your preference. This manual is a valuable resource for professionals seeking detailed guidance and insights. To get your copy, visit Amazon today and select the format that best supports your needs.
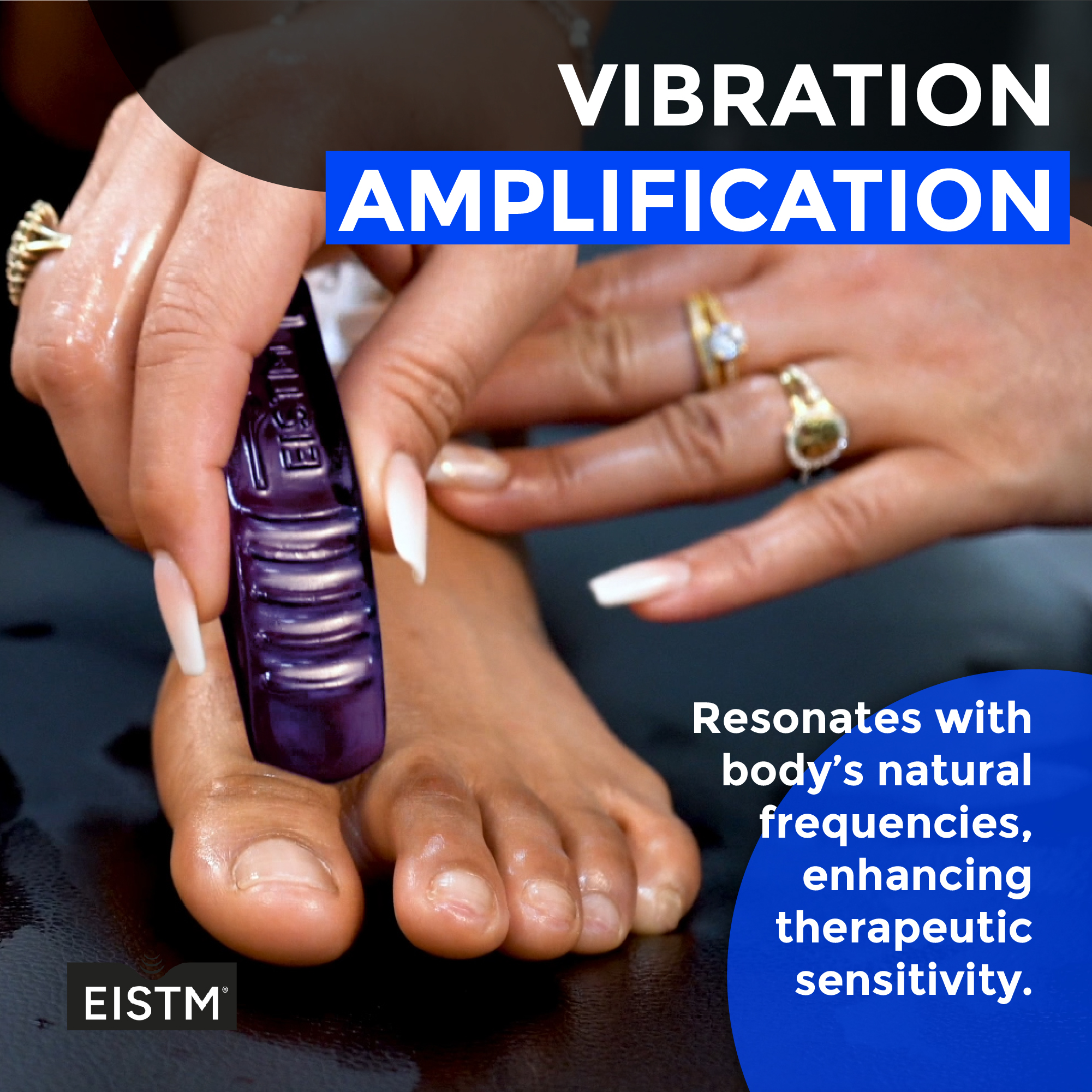
Need for improved tools, standards and practice
The shift towards third-generation tools in soft tissue mobilization marks a significant advancement in improving therapeutic outcomes, efficiency, and safety. The FDA determined that IASTM products are medical devices because they are intended to temporarily increase blood flow and local circulation at the site of application in healthy individuals. The FDA has determined that IASTM products are medical devices as defined in section 201(h) of the Federal Food, Drug, and Cosmetic Act and are general wellness products (class 1 medical device). The FDA has not approved or designated IASTM products for any purpose and has not made any determination about, or endorsement of, its stated use or benefits.




Third-generation tools are crafted to allow practitioners to feel adhesions through the instruments, enhancing diagnostic precision. Their design includes no-slip surfaces and 90-degree treatment edges, optimizing the manipulation of soft tissue decreasing discomfort or injury risk to the patient.
The objective is to notably enhance patient outcomes within a minimal number of visits, promoting a quicker return to normal activities and reducing the treatment burden.
These tools are available for outright purchase, eliminating the need for leasing, contracts, or hidden fees, thus ensuring a straightforward and cost-effective practice model.
Moreover, addressing hygiene concerns, particularly in minimizing cross-contamination, is paramount:
Transitioning to emollients in pump bottles significantly reduces contamination risks associated with traditional jar-based applications. This approach ensures a safer therapeutic environment by minimizing direct contact.
The recommended method for cleaning these new tools involves ultrasonic cleaners, which offer a thorough and efficient sanitization process. This method is capable of eliminating microorganisms that conventional cleaning methods may not, aligning with the stringent hygiene standards required in medical settings and ensuring the tools’ longevity and integrity.
This holistic approach combines therapeutic efficacy with the highest standards of hygiene and safety, representing a comprehensive advancement in patient care and practitioner experience in the field of soft tissue mobilization.
FDA Registration
IASTM, Gua Sha, and muscle scraping tools are classified as ‘Therapeutic Manual Massagers’ under Physical Medicine. These devices, with the product code LYG, are Class I and exempt from premarket review and regulated under number 890.5660 by the Office of Neurological and Physical Medicine Devices.

The administration of Gua Sha or Instrument-Assisted Soft Tissue Mobilization (IASTM) is generally performed by licensed healthcare professionals, depending on the regulations in the specific region or country. The following professionals are commonly allowed to administer Gua Sha or IASTM:
1. Physical Therapists (PTs) – Trained to perform IASTM as part of rehabilitative therapy.
2. Chiropractors – Often use IASTM techniques in their practice for musculoskeletal issues.
3. Occupational Therapists (OTs) – May use IASTM as part of hand therapy or other treatments.
4. Massage Therapists – Some are trained in Gua Sha or IASTM, depending on their certification and state regulations.
5. Acupuncturists – Frequently perform Gua Sha as part of traditional Chinese medicine practices.
6. Athletic Trainers – May be trained to use IASTM techniques, especially in sports settings.
7. Licensed Physicians – In some cases, physicians may also administer these techniques if they have received proper training.
The specific scope of practice and the legality of administering these techniques can vary widely, so it’s essential for practitioners to be licensed and certified according to their local regulations.
All medical devices sold in the United States are regulated by the U.S. Food and Drug Administration (FDA). Any device manufactured, repackaged, relabeled, or imported for sale in the U.S. must comply with FDA regulations. Devices that pass the 510(k) review process are considered ‘cleared,’ while those meeting safety and effectiveness standards in the PMA process are ‘approved.’ Once cleared or approved by the FDA, the device can be marketed for sale and use in the United States.
If you are aware of a medical device being marketed in the U.S. without FDA registration, you can report it to the FDA.
You can check their status on the FDA official website at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
You can report them directly at:
We stand by the quality of our EISTM tools and are committed to ensuring your satisfaction. All EISTM tools are warrantied against material defects for a period of one year from the date of purchase. If you experience any issues with your EISTM tool due to material defects within this period, we will gladly replace it free of charge.
To receive your replacement, simply cover the cost of shipping, and we’ll take care of the rest. Please contact our customer service team with your proof of purchase to initiate the replacement process.
Thank you for choosing EISTM.
We offer a flat shipping rate of $20.00 for deliveries anywhere within the 48 contiguous United States using USPS Ground Advantage.
For each tool set (whether a 3-piece or 5-piece set), the shipping cost is $20.00, with most packages arriving within 3-5 business days.
If you purchased the certification course, please email us your name and shipping address upon completion so we can send your certificate. The shipping fee is already included in your course fee.
If your package hasn’t arrived within a week of shipment, please contact us. We provide tracking numbers with every order, so you can monitor its progress. In the rare event of a lost package, the tracking number will help us assist you in locating it promptly.
Classified as Class | Medical Device by the FDA.


What is stereolithography?
Stereolithography (SLA) is a 3D printing technology, also known as optical fabrication, photo solidification, or resin printing. This process creates models, prototypes, patterns, and production parts layer by layer using light to initiate a photochemical reaction. This reaction causes chemical monomers and oligomers to bond, forming durable polymers that make up the structure of the three-dimensional object. SLA is renowned for its ability to produce highly accurate and high-resolution parts.
We are required to collect sales tax on every sale in accordance with state and local laws. The applicable sales tax will be automatically calculated and added to your total during the checkout process.
The exact amount of sales tax will vary based on your shipping address and will be displayed before you complete your purchase.
We offer the option to customize EISTM tools in your clinic’s preferred colors. To take advantage of this service, we require a minimum order of 5 sets.
Due to our use of advanced SLA stereolithography, please allow 2 weeks for production and an additional week for shipping. Simply provide us with your color specifications, and we’ll take care of the rest.
We want you to be completely satisfied with your purchase. If you need to return a product, we accept returns on items that are in unopened or like-new condition.
Please note that we reserve the right to charge a restocking fee of 20% of the product’s cost if the returned item has been mishandled or is materially damaged.
To initiate a return, please contact our customer service team with your order details. Once your return is approved, further instructions will be provided.

In the realm of additive manufacturing, the stereolithography process begins with the precise positioning of the build platform, which hovers mere millimeters above the surface of a tank brimming with liquid photopolymer resin. This setup is meticulously calibrated to a singular layer height.
Utilizing a UV laser, the process meticulously solidifies the resin, layer by intricate layer. This laser is directed by an advanced galvanometric system (galvo), which dictates the laser’s path with exceptional precision, ensuring each cross-sectional area of the model is scanned and solidified thoroughly, resulting in structurally robust components.
Following the formation of each layer, the build platform subtly ascends, allowing a sweeper blade to uniformly distribute a fresh layer of resin over the surface, thus preparing it for the subsequent layer. This cycle repeats seamlessly until the entire structure is fabricated.
Upon completion, the part exists in a “green” state—an intermediate phase where it is not fully cured. To achieve its maximum mechanical strength and thermal stability, the part undergoes a critical post-processing phase under UV light, which catalyzes the final polymerization and curing processes, enhancing the material properties and ensuring the component’s durability and functionality in demanding applications.
During this process, the liquid resin undergoes photopolymerization, where the UV light activates carbon chains in the monomer, turning the liquid into a solid. This creates strong, irreversible bonds, meaning the solidified resin cannot be reverted to its liquid form. Instead of melting when heated, these thermoset polymers will burn, unlike the thermoplastics used in FDM (Fused Deposition Modeling).
Technical Specifications Overview:
The Somos WaterShed XC 11122 material features a clear to translucent appearance with ABS-like properties, making it ideal for a variety of applications. It boasts a flexural strength of 69 MPa/KSI and an elongation at break of 15%, alongside a heat deflection temperature (HDT) at 0.46 MPa of 50°C. The standard resolution setup offers two build envelopes, with the large measuring 29” x 25” x 21” and the small 10” x 10” x 10”. Tolerances in the XY plane are maintained at +/- 0.005” for the first inch, with an additional +/- 0.002” for each subsequent inch, while in the Z plane, tolerances start at +/- 0.010” for the first inch, also adding +/- 0.002” per inch thereafter. Layer heights are set at 0.004” for the large build and 0.002” for the small. Feature resolution limits indicate that in the large build, features under 0.030” are at risk and those under 0.020” will not build, whereas in the small build, features under 0.020” are at risk and under 0.010” will not build. The minimum radial feature size is 0.035” for the large build and 0.030” for the small. These detailed parameters ensure that users can accurately assess the material’s suitability for complex designs, providing a reliable foundation for precision in advanced manufacturing applications.
EISTM Tools Safety and Usage Disclaimer
EISTM is committed to providing high-quality, reliable tools for therapeutic use. However, it is essential that all users understand and accept the responsibilities associated with the handling and use of these tools. EISTM is not liable for any injury, damage, or harm that may arise from the use of our tools. By purchasing and using EISTM tools, the purchaser agrees to the following conditions and acknowledges understanding of our safety guidelines:
Contraindications: EISTM tools are designed for professional use. They should not be used in the following situations:
• Over open wounds or infected areas
• By individuals with certain medical implants unless cleared by a healthcare provider
• On areas with severe varicose veins
• On areas receiving active cancer treatment
Liability: The purchaser assumes 100% responsibility for any injuries or damages that may occur due to the use of EISTM tools. This includes but is not limited to injury to self or others and damage to the tool or property.
Material Integrity: EISTM tools are made from high-quality SLA resin which is durable yet can be susceptible to damage if mishandled. Avoid dropping or striking the tools against hard surfaces. We recommend storing the tools in a secure, padded location when not in use.
-
Usage Guidelines
-
Handling Precautions
-
Alterations Prohibited
-
Liability Disclaimer
-
Safety and Use
-
Responsibility
• Always use EISTM tools as instructed by a trained professional.
• Do not expose the tools to temperatures above 175 degrees Fahrenheit (80 degrees Celsius), as extreme temperatures can affect the material properties.
• EISTM tools should be cleaned and sterilized according to standard medical practices to ensure they remain hygienic and safe for continuous use.
• When using EISTM tools, always wear appropriate protective gear such as gloves to prevent accidental injury.
• Ensure that all users are trained in the correct techniques to avoid misuse which could lead to injury or ineffective treatment.
Children and Vulnerable Individuals:
• EISTM tools are professional-grade instruments and should not be used by children or left within reach of children or vulnerable individuals without proper supervision.
• Do not attempt to modify, cut, drill, or heat the tools as this can compromise their structural integrity and safety.
• Disassembling or tampering with the tool in any way is strictly prohibited and will void the warranty.
By consenting to these terms and following these guidelines, you ensure a safer therapeutic environment for both practitioners and patients. Thank you for choosing EISTM tools for your therapeutic needs.
Rehabsurge 2, LLC. and any associated entities or individuals, including but not limited to staff, consultants, or affiliates, are not responsible or liable for any actions, services, or products acquired through this website or our training programs. We are also not responsible for any injury or harm that may result from the application of EISTM tools, regardless of the adherence to instructions or training provided.
While EISTM tools are designed to be safe when used correctly and with proper techniques, all users are advised to handle the tools with care. Users should:
• Ensure they are trained in proper techniques.
• Immediately cease use if the application of the tool becomes painful or if conditions worsen.
• Use the tools at their own risk, acknowledging that while every effort is made to ensure the safety and effectiveness of our tools, individual results and responses may vary.
By choosing to use EISTM tools, users accept full responsibility for any outcomes. It is recommended to address any questions or concerns with a medical professional to determine the suitability of the tools for your therapeutic needs.
EISTM is committed to providing quality therapeutic tools and educational resources to assist professionals in delivering effective care. However, users must acknowledge and accept the inherent risks associated with any therapeutic tool or intervention.
